OVERVIEW
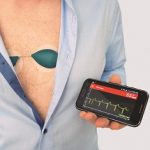
SAVVY is a lightweight, portable, clinical grade medical device designed to accurately monitor cardiac rhythm activity to provide early detection of problems.
WHAT CAN SAVVY DO?
SAVVY provides a comprehensive and reliable solution for monitoring heart activity for the early detection of various heart conditions as well as tracking the heart during advanced exercise.
Instead of spending time in an clinic wearing a traditional heart monitor apparatus, SAVVY allows patients to follow their normal routine whilst their heart activity is being constantly monitored and recorded.
HOW DOES SAVVY WORK?
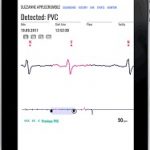
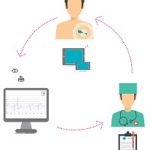
SAVVY is placed on to the chest with two ECG electrodes. The measured data is sent via a radio connection to your smart device (a phone or tablet1) which stores the ECG2 record. This data can then be transferred to your computer and printed out or sent by email (real-time) to your medical professional.
WHY CHOOSE SAVVY?
SAVVY weighs only 21 grams and is designed to not interfere with your movements. It is biocompatible and skin-friendly, water resistant to 1m so it can be worn in the shower to provide a continous set of accurate data. The unit has a battery life of 7 days, can be used continuously for at least two years and due to its small size, is unobtrusive under clothing. SAVVY produces high fidelity signals and is manufactured to meet clinical requirements.
WHO USES SAVVY?
The SAVVY diagnostic cardiac monitor is an indispensable device for most chronic patients and those that feel an occasional irregular heartbeat.
It is available without prescription, so can be used by advanced recreational and professional athletes. SAVVY does not only show average values but records every heartbeat, the foundation of a precise heart activity analysis.
Some popular uses are:
- Athletes monitoring issues that may be a result of undiagnosed arrhythmia
- Business people tracking heart activity following a stroke or abnormal heart rhythm
- Elderly patients whose medical advisor wishes to assess the affect of medication on heart activity
WHY TRUST SAVVY?
SAVVY is CE approved as a medical device according to EU directives 93/42/EEC and also according to ISO 13485:2003 for quality management systems for medical devices and IEC 62304:2006 for medical software development.
1 Apple, Android
2 Electrocardiogram – a graphic readout of heart muscle activity